The Helium Bohr Model: Ultimate Guide to Understanding Atomic Structure 2026

Introduction
Have you ever wondered what makes helium so special at the atomic level? The helium Bohr model gives us a fascinating window into the atomic world. It’s one of the simplest yet most important concepts in chemistry and physics.
When Niels Bohr introduced his atomic model in 1913, he revolutionized how we understand atoms. While he initially focused on hydrogen, the helium Bohr model became crucial for understanding multi-electron systems. You’ll find this model explains why helium behaves the way it does.
In this guide, I’ll walk you through everything about the helium Bohr model. You’ll learn how electrons orbit the nucleus, why energy levels matter, and how this model shaped modern atomic theory. Whether you’re a student or just curious about atomic structure, this article makes it simple.
What Is the Helium Bohr Model?
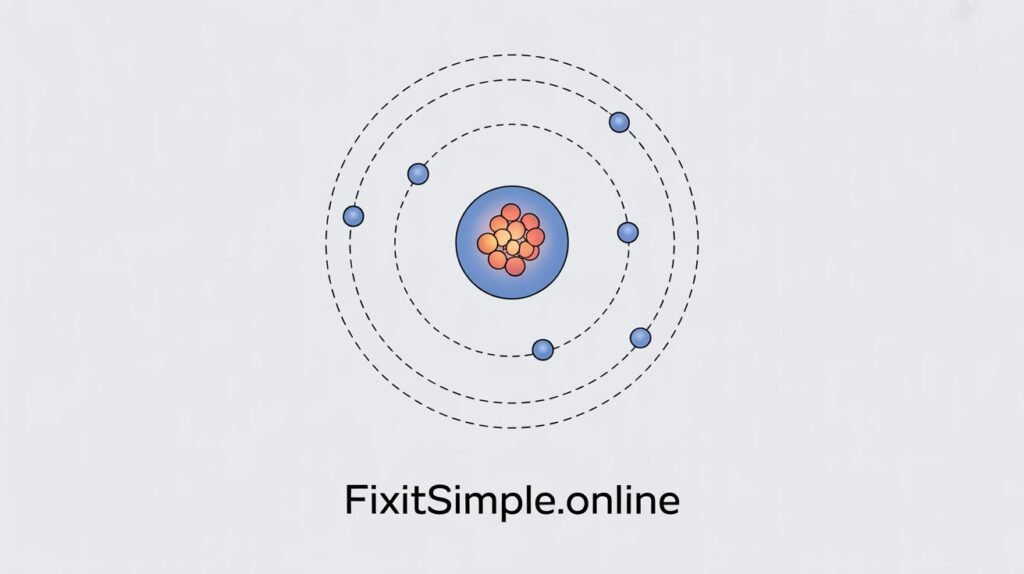
https://fixitsimple.online/the-scientist-lyrics-meaning/The helium Bohr model represents helium’s atomic structure using Niels Bohr’s theoretical framework. It shows how two electrons orbit around a nucleus containing two protons and two neutrons.
Think of it like a miniature solar system. The nucleus sits at the center, acting like the sun. The electrons orbit around it in specific paths called shells or energy levels.
Basic Components of the Helium Atom
The helium atom consists of three main parts:
- Nucleus: Contains 2 protons (positive charge) and typically 2 neutrons (neutral)
- Electrons: Two negatively charged particles orbiting the nucleus
- Energy levels: Specific orbital paths where electrons exist
The atomic number of helium is 2. This means it has two protons in its nucleus. To maintain electrical neutrality, it also has two electrons.
Why the Helium Bohr Model Matters
Understanding the helium Bohr model helps you grasp fundamental chemistry concepts. It explains chemical stability, noble gas behavior, and electron configuration patterns.
Helium is the second element on the periodic table. It’s also the first noble gas. The helium Bohr model shows why helium rarely reacts with other elements.
Historical Background of the Bohr Model
Niels Bohr, a Danish physicist, proposed his atomic model in 1913. He was trying to solve problems that classical physics couldn’t explain.
Before Bohr, scientists used the Rutherford model. Ernest Rutherford discovered the nucleus in 1911. But his model had a major flaw—it couldn’t explain atomic stability.
The Problem Bohr Solved
Classical physics predicted that orbiting electrons should lose energy continuously. They would spiral into the nucleus within nanoseconds. Obviously, atoms don’t collapse this way.
Bohr introduced a revolutionary idea. He proposed that electrons orbit only in specific energy levels. Electrons don’t radiate energy when they’re in these stable orbits.
Bohr’s Key Postulates
Bohr built his model on three main ideas:
- Quantized orbits: Electrons exist only in specific circular orbits
- Stationary states: Electrons don’t emit radiation in these orbits
- Quantum jumps: Electrons absorb or emit energy when jumping between levels
These postulates worked brilliantly for hydrogen. But when Bohr applied them to the helium Bohr model, things got more complex.

Structure of the Helium Bohr Model
In the helium Bohr model, both electrons occupy the first energy level. This is called the K shell or n=1 shell.
The first energy level can hold a maximum of 2 electrons. Helium’s electron configuration is 1s², meaning both electrons fill the first shell completely.
Electron Arrangement
Both electrons orbit the nucleus at roughly the same distance. They move in the same energy level but in different orbital patterns to minimize repulsion.
The electrons maintain opposite spins. This follows Pauli’s exclusion principle, which states that no two electrons can have identical quantum states.
Distance from Nucleus
In the helium Bohr model, electrons orbit at approximately 0.529 angstroms from the nucleus. This is called the Bohr radius for the first energy level.
The actual distance is slightly smaller than in hydrogen. Why? Because helium’s nucleus has two protons instead of one. The stronger positive charge pulls electrons closer.
Energy Level Characteristics
The first energy level in helium has specific properties:
- Principal quantum number: n = 1
- Maximum electrons: 2
- Orbital type: s-orbital (spherical)
- Energy state: Lowest possible (ground state)
When both electrons occupy this level, helium achieves maximum stability. This explains why helium is chemically inert.
Energy Levels in the Helium Bohr Model
Energy levels determine where electrons can exist around the nucleus. Think of them as specific rungs on a ladder. Electrons can stand on these rungs but nowhere in between.
In the helium Bohr model, we focus primarily on the first energy level. Both electrons reside here in helium’s ground state.
Calculating Energy Levels
Bohr derived a formula for energy levels: E = -13.6 eV × (Z²/n²)
Where:
- E = energy of the level
- Z = atomic number (2 for helium)
- n = principal quantum number (1, 2, 3…)
For helium’s first energy level: E = -13.6 × (4/1) = -54.4 eV
This negative value indicates that energy must be added to remove an electron. The electron is bound to the nucleus.
Ionization Energy
Helium has the highest first ionization energy of all elements. It requires 24.6 eV to remove one electron from neutral helium.
This high ionization energy makes helium extremely stable. You need significant energy to disrupt the helium Bohr model’s electron configuration.
Excited States
Although helium’s electrons normally occupy the first level, they can jump to higher levels. This happens when helium absorbs energy from heat, electricity, or light.
When an electron jumps to a higher level, helium enters an excited state. This state is temporary. The electron eventually falls back down, releasing energy as light.
This principle explains how neon signs work, even though they’re filled with various gases including helium.
Limitations of the Helium Bohr Model
The helium Bohr model works reasonably well for basic understanding. But it has significant limitations when you dig deeper.
Electron-Electron Repulsion
Bohr’s original model didn’t account for electron-electron repulsion. In helium, two electrons occupy the same energy level. They repel each other due to their negative charges.
This repulsion affects the actual energy levels. Calculations become much more complex than the simple Bohr formula suggests.
Multi-Electron Complications
The Bohr model works best for hydrogen-like systems. These are atoms or ions with only one electron.
For the helium Bohr model, we have two electrons. They interact with each other and the nucleus simultaneously. Classical Bohr theory can’t handle these three-body interactions accurately.
Spectral Line Predictions
When scientists examined helium’s spectral lines, they found discrepancies. The Bohr model predicted certain wavelengths of light. But actual measurements showed additional lines and split peaks.
These observations revealed that the helium Bohr model was too simplified.
The Quantum Mechanical Solution
Modern quantum mechanics replaced Bohr’s model in the 1920s. Scientists like Schrödinger and Heisenberg developed more accurate theories.
Quantum mechanics treats electrons as wave-particles, not tiny planets. It uses probability clouds instead of fixed orbits. This approach accurately predicts helium’s properties.
However, the helium Bohr model remains valuable for teaching. It provides an intuitive stepping stone to more complex theories.
Comparing Hydrogen and Helium Bohr Models
The hydrogen Bohr model features one proton and one electron. It’s the simplest atomic system possible.
The helium Bohr model adds complexity with two protons, two neutrons, and two electrons. This additional electron creates new challenges.
Key Differences
Here’s how they compare:
| Feature | Hydrogen | Helium |
|---|---|---|
| Protons | 1 | 2 |
| Electrons | 1 | 2 |
| Nuclear charge | +1 | +2 |
| Electron repulsion | None | Present |
| Ionization energy | 13.6 eV | 24.6 eV |
| Reactivity | Reactive | Inert |
Why Helium Is More Stable
The helium Bohr model shows a completely filled first energy level. This configuration is extremely stable.
Hydrogen has an incomplete first shell. It has room for one more electron. This makes hydrogen chemically reactive—it wants to gain, lose, or share that electron.
Mathematical Complexity
Calculating hydrogen’s properties using Bohr’s equations is straightforward. You can solve it with basic algebra.
The helium Bohr model requires more advanced mathematics. The electron-electron repulsion term makes exact solutions impossible using Bohr’s approach.
Applications of the Helium Bohr Model
Even with its limitations, the helium Bohr model has practical applications. It helps us understand real-world phenomena.
Noble Gas Behavior
The helium Bohr model perfectly illustrates noble gas stability. A filled outer shell means no need to react with other elements.
This explains why helium doesn’t form compounds under normal conditions. It’s already in its most stable configuration.
Balloons and Airships
We use helium in balloons because it’s lighter than air. But we also choose it for safety—it won’t react or burn.
The helium Bohr model shows why. Those two electrons sitting snugly in the first shell won’t participate in chemical reactions.
Cooling and Cryogenics
Liquid helium serves as a super-coolant in scientific equipment. MRI machines rely on liquid helium to cool superconducting magnets.
Helium’s atomic properties, visible in the helium Bohr model, contribute to its ultra-low boiling point (-269°C).
Spectroscopy
Scientists use helium spectral lines to study stars and distant galaxies. The specific wavelengths helium emits act like a fingerprint.
Understanding the helium Bohr model helps astronomers interpret these spectral signatures. They can determine the composition of celestial objects millions of light-years away.
Educational Value
Perhaps most importantly, the helium Bohr model teaches fundamental concepts. It bridges the gap between simple atomic theory and complex quantum mechanics.
Students worldwide learn about electron shells, energy levels, and atomic stability through this model.
How to Draw the Helium Bohr Model
Drawing the helium Bohr model is straightforward. You’ll need paper, a pencil, and optionally colored markers.
Step-by-Step Instructions
- Draw the nucleus: Make a small circle in the center of your page
- Label the nucleus: Write “2p+ 2n” inside (2 protons, 2 neutrons)
- Draw the first energy level: Create a larger circle around the nucleus
- Add electrons: Draw two small circles or dots on the orbital path
- Label electrons: Mark each with “e-” or use a different color
- Add details: Include labels for “Nucleus,” “First Energy Level,” and “Electrons”
Common Mistakes to Avoid
Don’t draw electrons at opposite sides of the orbit. While they do repel each other, their exact positions change constantly.
Don’t add a second energy level. Both helium electrons stay in the first shell.
Don’t forget the neutrons. While they don’t affect the Bohr model’s basic structure, they’re part of the complete picture.
Color Coding
I recommend using colors to make your diagram clearer:
- Red or orange: Protons (positive charge)
- Gray or white: Neutrons (neutral)
- Blue or green: Electrons (negative charge)
This color scheme helps viewers quickly identify different particles.
Helium Bohr Model vs. Modern Quantum Model
The helium Bohr model depicts electrons as particles in circular orbits. The quantum mechanical model treats them very differently.
Electron Clouds vs. Orbits
Quantum mechanics doesn’t show fixed paths. Instead, it calculates probability distributions. These create “clouds” where electrons are likely to exist.
For helium, the quantum model shows a spherical 1s orbital. Both electrons occupy this orbital with opposite spins.
Accuracy Comparison
The Bohr model predicts helium’s ionization energy as 54.4 eV. The actual measured value is 24.6 eV. That’s a significant error.
Quantum mechanics calculates 24.5 eV. This matches experimental results almost perfectly.
When to Use Each Model
Use the helium Bohr model when you need:
- Simple visual representation
- Basic understanding of electron shells
- Quick explanations for beginners
- Foundational chemistry concepts
Use quantum mechanics when you need:
- Precise energy calculations
- Detailed spectroscopic predictions
- Advanced research applications
- Professional chemistry or physics work
The Bridge Between Models
The helium Bohr model isn’t wrong—it’s incomplete. It captures essential truths about atomic structure. Energy levels exist. Electrons do occupy specific shells. Stability comes from filled shells.
Quantum mechanics adds layers of complexity and accuracy. But it builds on foundations that Bohr established.
Teaching the Helium Bohr Model
If you’re explaining the helium Bohr model to others, start with familiar concepts. Compare the atom to a solar system. Use visuals extensively.
Breaking Down Complex Ideas
Don’t introduce everything at once. Start with the nucleus. Then add one electron. Finally, add the second electron and discuss repulsion.
This sequential approach prevents overwhelm. It mirrors how scientists historically developed atomic theory.
Hands-On Activities
Students retain information better through active learning. Here are some ideas:
- Build 3D models: Use balls and wire to create physical helium Bohr models
- Interactive simulations: Online tools let students manipulate energy levels
- Drawing exercises: Have students sketch and label their own diagrams
- Comparison charts: Create tables comparing hydrogen and helium
Common Student Questions
“Why don’t electrons fall into the nucleus?” Explain Bohr’s postulate about stable orbits. Energy quantization prevents the spiral collapse.
“How do electrons know where to orbit?” This question reveals the model’s limitations. Transition to quantum mechanics here if appropriate.
“Can helium ever have electrons in the second shell?” Yes, in excited states. Explain energy absorption and emission.
The Future Beyond Bohr
Atomic theory continues evolving. The helium Bohr model represents one chapter in this ongoing story.
Current Research
Scientists now use quantum computers to model complex atoms. These simulations go far beyond what Bohr imagined.
Researchers study exotic helium isotopes like helium-3. They explore how different nuclear configurations affect electron behavior.
Helium in Quantum Computing
Helium plays a role in quantum computing research. Its stability and well-understood properties make it useful for testing quantum theories.
Some quantum computers operate at temperatures approaching absolute zero. Liquid helium makes this possible.
Educational Evolution
How we teach atomic structure keeps changing. Virtual reality might soon let students “fly” through a helium Bohr model. They could watch electrons orbit in three dimensions.
But the fundamental concepts remain valuable. Understanding the helium Bohr model still forms a crucial foundation for advanced study.
Conclusion
The helium Bohr model offers an elegant introduction to atomic structure. It shows how electrons, protons, and neutrons create the second element on the periodic table.
You’ve learned how two electrons occupy helium’s first energy level. This filled shell configuration explains helium’s stability and inert nature. You understand why Bohr’s model works well for basic concepts but falls short for precise calculations.
While modern quantum mechanics provides greater accuracy, the helium Bohr model remains invaluable. It teaches essential principles of electron shells, energy levels, and atomic stability. These concepts underpin all of chemistry and much of physics.
Whether you’re a student tackling chemistry homework or someone curious about the atomic world, the helium Bohr model gives you a solid starting point. From here, you can explore deeper into quantum mechanics or apply these basics to understanding other elements.
What aspect of the helium Bohr model fascinates you most? Share your thoughts and keep exploring the amazing world of atomic structure!

FAQs About the Helium Bohr Model
Q1: How many electrons does the helium Bohr model show?
The helium Bohr model shows two electrons orbiting the nucleus. Both electrons occupy the first energy level (K shell) in helium’s ground state. This completely fills the first shell, which can hold a maximum of two electrons.
Q2: Why is the helium Bohr model important for understanding chemistry?
The helium Bohr model illustrates the concept of a filled electron shell. This configuration explains chemical stability and noble gas behavior. It serves as a foundational example for understanding how electron arrangement determines an element’s chemical properties.
Q3: What are the limitations of the helium Bohr model?
The helium Bohr model cannot accurately account for electron-electron repulsion. It treats electrons as particles in fixed orbits rather than wave-particles. The model also fails to predict helium’s spectral lines precisely. Quantum mechanics provides more accurate descriptions of multi-electron atoms.
Q4: How does the helium Bohr model differ from hydrogen’s?
The helium Bohr model has two electrons instead of one, creating electron-electron repulsion. Helium’s nucleus has twice the positive charge, pulling electrons closer. Helium achieves a stable filled shell, while hydrogen has an incomplete outer shell. These differences make helium inert and hydrogen reactive.
Q5: Can the helium Bohr model explain why helium is a noble gas?
Yes, the helium Bohr model clearly shows why helium is a noble gas. Both electrons completely fill the first energy level. Atoms are most stable with filled outer shells. Since helium’s outer shell is full, it doesn’t need to gain, lose, or share electrons.
Q6: What is the energy of helium’s first energy level in the Bohr model?
Using Bohr’s formula E = -13.6 eV × (Z²/n²), where Z=2 for helium and n=1, the calculated energy is -54.4 eV. However, this doesn’t match experimental measurements due to electron-electron repulsion. The actual first ionization energy is 24.6 eV.
Q7: How do you draw the helium Bohr model accurately?
Draw a small central circle for the nucleus labeled with 2 protons and 2 neutrons. Draw a larger circle around it representing the first energy level. Place two electrons on this orbital circle. Don’t add additional energy levels since both electrons occupy only the first shell.
Q8: Does the helium Bohr model show neutrons?
Yes, a complete helium Bohr model includes neutrons in the nucleus. Helium-4, the most common isotope, has two neutrons. While neutrons don’t affect electron orbits directly, they contribute to atomic mass and nuclear stability. They’re important for a comprehensive atomic representation.
Q9: When was the helium Bohr model developed?
Niels Bohr developed his atomic model in 1913, initially for hydrogen. Application to the helium Bohr model came shortly after. However, scientists quickly realized that Bohr’s equations didn’t work as precisely for helium’s two-electron system. This spurred further development of quantum theory.
Q10: Is the helium Bohr model still used today?
The helium Bohr model is still widely used in education. It provides an intuitive introduction to atomic structure and electron shells. However, professional scientists use quantum mechanical models for research and accurate predictions. The Bohr model serves as a useful simplified representation rather than a precise scientific tool.
Also read fixitsimple.online